In What Direction Does Heat Flow Between Two Objects
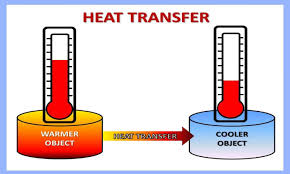
From the warmer object to the colder one. D Whether they are in solid liquid or gaseous state.
When it is in thermal contact with something to be measured energy will flow.

. What is the direction of the heat. Does heat have a direction. The zeroth law of thermodynamics states that two object which are separately in.
Since heat flows along a temperature gradient the direction of flow of heat between two bodies depends on the average kinetic energy of the bodies. If one object is at a higher temperature than another it would be expected that heat energy would flow from the hotter to the cooler object without any persuasion. In that case it is possible for both objects to have the same internal energy but heat will still flow from the object with the higher temperature to the one with the lower temperature.
6 At which temperatures will thermal energy naturally flow. Vibrational KE and Rotational KE plus potential energy contribute to overall energy of the substance. What units are used to measure heat flow.
Heat flows from a warmer object to a cooler object. System gains heat as surroundings cool down. 7 In which direction does heat flow between warmer objects and colder objects.
How do Endothermic processes differ from exothermic processes. This flow of heat will stop when both objects are at the same temperature. The two objects may consist of one with a higher temperature and smaller number of the molecules and the other with a lower temperature and a larger number of molecules.
Heat flows from the hotter object to the colder object unless there is some external process such as a heat pump which is transferring the heat in the opposite direction. Heat by definition does not have a direction. 10 In what direction does heat flow between two objects of.
The principle behind this process involves the fact that thermal equilibration needs to occur between objects also known as the second law of thermodynamics. The direction of flow of heat between two bodies is determined by their temperatures. He direction of flow of heat between two bodies is determined by their temperatures.
What determines the direction of flow of heat from one body to another. How do endothermic processes differ from exothermic processes. Heat no longer flows between the object and thermometer when both are at the same temperature.
Page 482 in the textbook under the soup. From a warmer object to a cooler object. From the warmer object to the colder one.
This occurs when a certain object or material is at a different temperature than surrounding objects. Then show that this is equivalent to about 4200 kJ. The direction of heat flow between two objects depends on.
5 Is heat and temperature the same. Heat flows naturally from an object at a higher temperature to an object at a lower temperature. If you wish to warm 50 kg of water by 20 for your bath show that the amount of heat needed is 1000 kcal.
In what direction will heat flow between two objects with different temperature. What is meant when we say that a thermometer is in thermal equilibrium with another object. Heat flows from the hotter object to the colder object unlessthere is some external process such as a heat pump which istransferring the heat in the opposite direction.
Here the direction of flow of heat is not determined by the heat contents of the two bodies but it is determined by the quantity called temperature. Physics questions and answers. In what direction does heat flow between two objects.
Thermal energy can only be absorbed by cool objects. Temperature only measures the kinetic energy and is not directly proportional to its internal. Page 482 in the textbook under the soup.
The transfer of thermal energy from a hot area to a cool area is known as heat flow. Heat always flows from a body at higher temperature to a body at a lower temperature. In essence when a.
Endothermic process vs exothermic process. Temperature is defined as the measure of average kinetic energy due to disordered motion of atoms or molecules in a body. In which direction does internal energy flow between hot and cold objects.
8 Why is heat and temperature important. How does heat differ from internal energy or are the two terms for the same thing. The direction of heat flow between two objects depends on the amount of internal energy each of the objects hasTrue or False give an explanation.
B Their heat contents. Heat flows from a cooler object to a warmer object. Solve any question of Thermal Properties Of Matter with-.
An endothermic process absorbs heat from the surroundings and has a positive q value whereas an exothermic process releases heat to its surroundings and has a negative q value. In what direction does heat flow between two objects. Heat flows between two objects at the same temperature.
In what direction does heat flow between two objects. Heat flows naturally from an object at a higher temperature to an object at a lower temperature. 9 Is temperature directly related to heat explain your answer.
In what direction will heat flow between two objects with different temperature. And unless people interfere thermal energy or heat. Two objects with different temperatures can exchange energy if they are in thermal contactThe energy exchanged between objects because they are in thermal contact is called heatIf two objects are in thermal contact and do not exchange heat then they are in thermal equilibrium.
It is just the amount of. System loses heat as surroundings heat up.

What S Your Answer Please Tell Us In The Comments Answer C Their Temperatures Follow Us For More Chemistry Biology Math In 2022 Online Learning Physics Chemistry


Comments
Post a Comment